The Global Need for Affordable, Early Cancer Diagnostics
OncoFirm Diagnostics | https://oncofirmdiagnostics.com
1. Executive Summary
Early detection of cancer remains one of the most cost-effective, life-saving interventions in modern medicine. However, millions globally still lack access to timely, affordable diagnostics. OncoFirm Diagnostics aims to revolutionize early cancer screening through rapid, antigen‑based lateral flow tests for underserved regions—bridging critical access gaps and reducing mortality.
2. The Cancer Challenge: Scale & Impact
- In 2020, there were ~19.3 million new cancer cases and nearly 10 million deaths worldwide Facebook+8LinkedIn+8oncofirm.com+8ACS Journals+1SpringerLink+1.
- Though cancers detected at an early stage have treatment success rates as high as 90% (e.g., breast cancer), approximately 50% are still diagnosed late .
- The global multi‑cancer early detection market was USD 1.12 billion in 2024 and is projected to grow to USD 2.86 billion by 2030 (CAGR ~17%) oncofirmdiagnostics.com+10Grand View Research+10ITIF+10.
- Solid tumor diagnostic demand reached USD 20.5 billion in 2023, with a 7.6% annual growth expected through 2032 MLO Online.
2.1 Disparities in Diagnostic Access
- Low‑ and middle‑income countries (LMICs) account for ~70% of cancer deaths, yet fewer than 30% have basic diagnostic facilities PMC+15Financial Times+15LinkedIn+15.
- Barriers like cost, infrastructure, and lack of trained professionals lead to late diagnoses and poor outcomes Grand View Research+1arXiv+1.
3. Value of Early Detection
- Public health data have shown up to a 70% reduction in mortality from cervical cancer due to Pap smears, and a 25% decrease in breast cancer deaths from mammography ITIF.
- Economic analysis estimates national cost‑savings in the U.S. into the billions from earlier diagnosis of breast, lung, prostate, and colorectal cancers arXiv.
- Technological strides like liquid biopsies and AI‑driven diagnostics are enabling affordable, point‑of‑care screening Facebook+15PMC+15LinkedIn+15.
4. Limitations of Current Screening
- Only five cancers (breast, cervical, colorectal, prostate, lung) have widespread guideline-recommended screening; most others remain undetectable until late stage ITIF.
- Existing diagnostic tools are costly, lab-bound, and inaccessible in many regions—leading to diagnosis delays, incomplete care, and health inequity .
5. OncoFirm’s Innovation: Affordable, Ultra-Rapid, Accessible
5.1 Proprietary Antigen-Based LFA & FLFT Tests
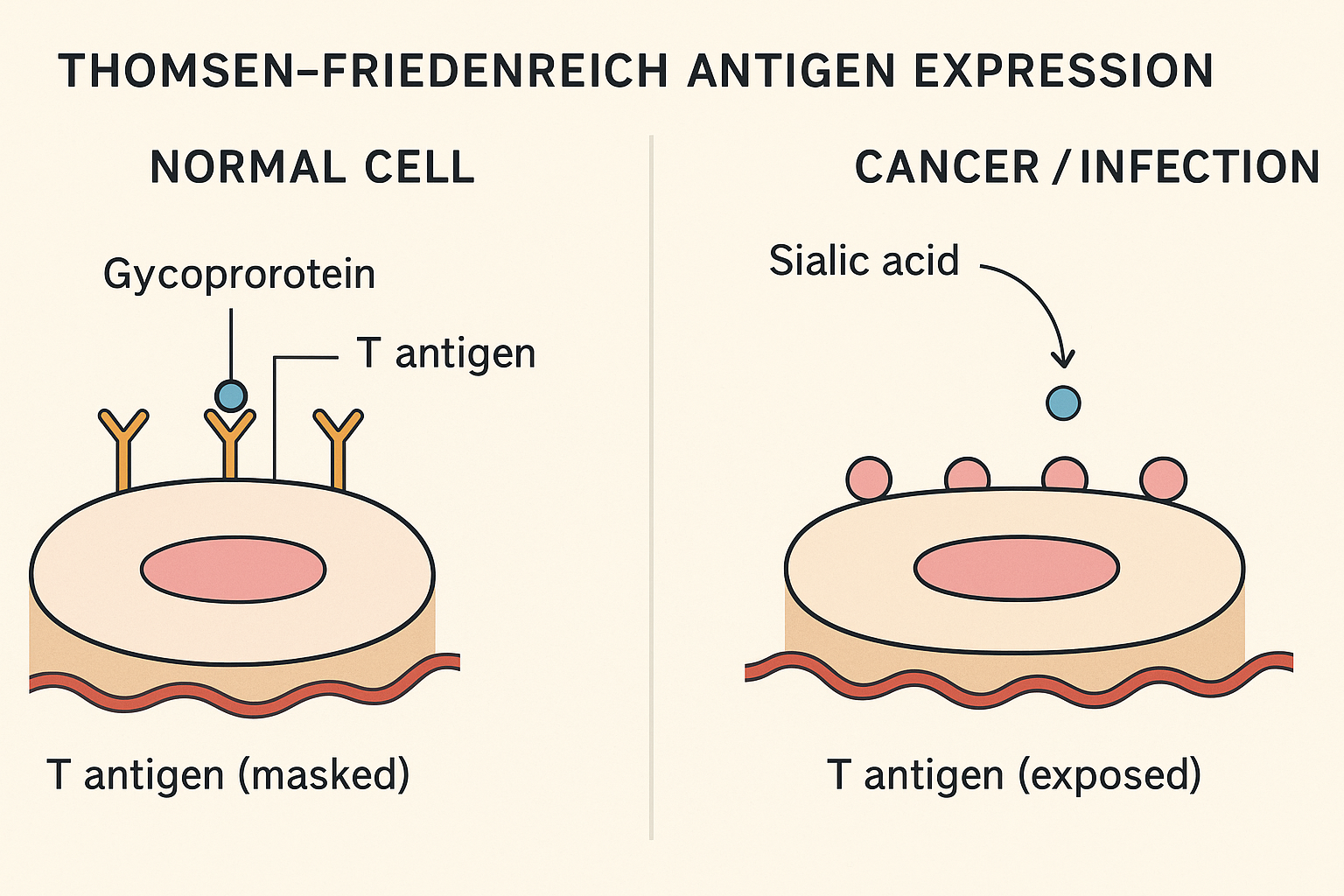
- OncoFirm combines fluorescent and traditional lateral flow assay (LFA) technology using novel cancer‑specific antigens (e.g., Thomsen–Friedenreich polysaccharides) for high-sensitivity biomarker detection Facebook+8oncofirmdiagnostics.com+8LinkedIn+8.
- Panel includes multiplex tests: breast, colorectal, lung, prostate, liver, ovarian, pancreatic, GI cancers.
5.2 Point‑of‑Care Deployment
- Results in under 15 minutes, with no lab needed—ideal for remote, clinic, or resource-limited settings Rapid Cancer Screening+1LinkedIn+1.
5.3 Global Reach & Regulatory Path
- Premarket stage: CE‑marking targeted for Europe, Latin America, and Asia; FDA submission underway LinkedIn+1MLO Online+1.
- Founded in 2025, based in Delaware (US), launched Q2‑2025 with up to $10M in seed funding LinkedIn+1LinkedIn+1.
6. Market Opportunity
- Multi-cancer early detection market poised to reach USD 2.86 billion by 2030 Grand View Research+1ITIF+1.
- OncoFirm’s rapid, low-cost format aligns with needs in LMICs, bridging the 70% of cancer deaths occurring in those regions PMC+15Financial Times+15Rapid Cancer Screening+15.
- Solid tumor testing demand further supports growth, with increasing global awareness and public investments MLO Online+1Grand View Research+1.
7. Strategic Advantages
| Feature | Impact |
|---|---|
| Speed & Ease | Results in 10–20 minutes; no labs needed Rapid Cancer Screening+1Early Cancer Rapid Detection Tests+1 |
| Low Cost | Affordable antigen-based format boosts scalability |
| Scalable Multiplex Panels | Covers multiple cancers per test |
| Regulatory & Funding Traction | CE process and $10M seed round underway LinkedIn |
8. Implementation Roadmap
- Clinical Validation & CE Approval – Target 2025–2026.
- Manufacturing Scale-Up – Establish global supply chains.
- Strategic Market Entry – Deploy in Europe, Asia, Latin America.
- FDA Submission – Begin US market access.
- Partnerships – Collaborate with NGOs, ministries of health, and diagnostics alliances (e.g., FIND) to expand distribution in LMICs LinkedInen.wikipedia.org.
9. Conclusion
OncoFirm Diagnostics is uniquely positioned to address one of the most pressing global health challenges: the inequity in access to early cancer diagnostics. By delivering rapid, precise, and affordable antigen-based tests—unbound by labs—OncoFirm can transform cancer care in places that need it most, drive down mortality, and align with global health priorities.
Investors, public health agencies, and healthcare providers are invited to partner in scaling this scalable, high-impact solution—making early cancer detection the global standard, not a luxury.
10. References
See inline citations throughout the whitepaper.