RNA Polymerase II Levels in Histone Genes: A Predictive Biomarker for Tumor Aggressiveness and Recurrence
RNA Polymerase II Levels in Histone Genes: A Predictive Biomarker for Tumor Aggressiveness and Recurrence
Introduction
Histone genes play a fundamental role in DNA packaging and chromatin structure, and their expression is tightly regulated during the cell cycle, particularly in S-phase. RNA Polymerase II (RNAPII), the enzyme responsible for transcribing protein-coding genes, including replication-dependent histone genes, has emerged as a key player in gene regulation dynamics during cancer progression. Recent research has identified a significant link between hyper-elevated RNAPII occupancy on histone genes and tumor aggressiveness, providing insights into both cancer biology and potential therapeutic interventions.
Findings Summary
Researchers have discovered that elevated RNAPII levels on histone genes are not merely reflective of increased gene transcription, but are also strongly correlated with poor cancer prognosis, including:
- Tumor aggressiveness
- Increased recurrence rates
- Genomic instability
These observations suggest that RNAPII may act beyond a passive transcriptional role, potentially contributing to chromosomal changes that facilitate tumor progression.
Mechanistic Insights
1. Transcriptional Dysregulation of Histone Genes
Histone mRNAs are unique in that they lack poly(A) tails and are processed differently. Their expression is synchronized with DNA replication to ensure proper chromatin assembly. The accumulation of RNAPII on these genes suggests:
- Excessive transcriptional activity, potentially leading to aberrant histone protein production.
- Dysregulation of chromatin structure, which can disrupt genomic integrity.
2. Connection to Chromosomal Instability
Aberrant histone levels can alter nucleosome density and positioning, possibly creating:
- Replication stress
- Increased DNA damage
- Susceptibility to chromosomal translocations
These effects are often precursors to oncogenic transformation and tumor evolution.
3. RNAPII as a Stress Marker
Hyper-accumulation of RNAPII on histone loci may indicate persistent S-phase transcriptional stress, a hallmark of high proliferation rates observed in aggressive tumors. This supports the hypothesis that elevated RNAPII levels may reflect intrinsic replication stress or oncogene activation.
Clinical Implications
1. Biomarker Potential
Given the correlation between elevated RNAPII and cancer severity:
- RNAPII levels at histone gene loci could serve as a prognostic biomarker, allowing early identification of high-risk patients.
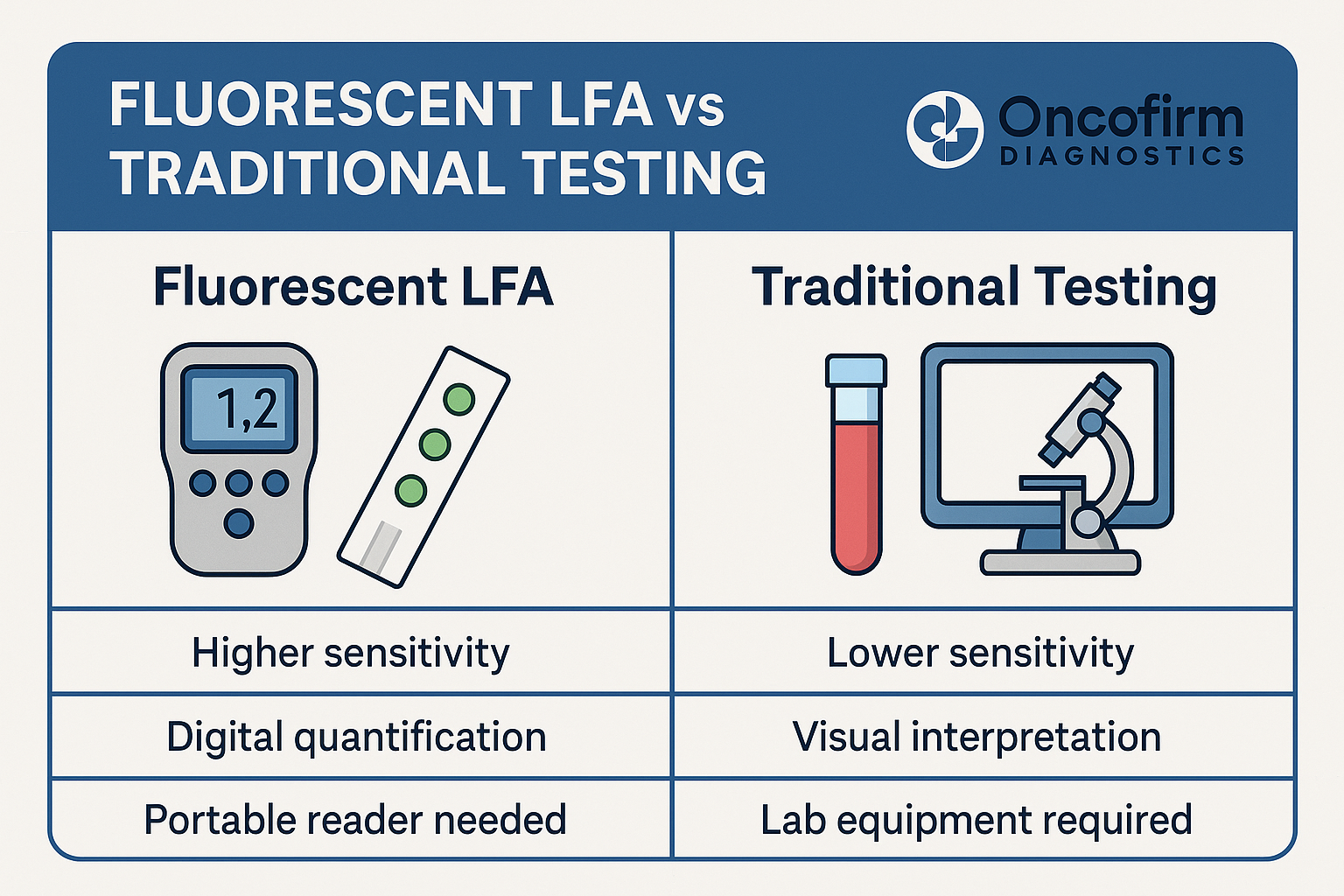
- Measurement of RNAPII occupancy through techniques like ChIP-seq or RNAPII-specific CUT&RUN could be integrated into diagnostic pipelines.
2. Therapeutic Targeting
If RNAPII accumulation contributes directly to tumor progression:
- Targeting RNAPII activity with transcriptional inhibitors (e.g., CDK7/9 inhibitors) could selectively impair hyper-proliferative cancer cells.
- Combination therapies could be designed to exploit this vulnerability in tumors with high RNAPII-histone gene activity.
Challenges and Future Directions
Despite the promising findings, several areas warrant further investigation:
- Causal Relationships: Is RNAPII elevation driving tumorigenesis, or is it a consequence of other oncogenic processes?
- Histone Gene Specificity: Do specific histone variants or clusters show stronger associations with RNAPII elevation?
- Tumor Type Variation: How consistent is this biomarker across various cancers—e.g., breast, prostate, glioblastoma?
Future research may also explore:
- The epigenetic landscape surrounding histone gene loci with high RNAPII levels.
- RNAPII post-translational modifications that may influence its activity and binding patterns in cancer cells.
Conclusion
The discovery of hyper-elevated RNAPII levels on histone genes as a marker of tumor aggressiveness and recurrence opens a novel window into cancer diagnostics and treatment. RNAPII not only reflects transcriptional output but may actively influence chromosomal dynamics and genomic stability. As research progresses, RNAPII could emerge as both a biomarker for cancer prognosis and a therapeutic target in precision oncology.